Who We Are
Footprint
Today and the Future
Recordati is uniquely structured to bring treatment options across specialty and primary care, consumer healthcare, and rare diseases. We have fully-integrated operations across research & development, chemical and finished product manufacturing through to commercialisation and licensing.
We are continually growing, with a focus on developing new specialties, new treatments and investing in medical innovations that bring a brighter future to patients across the world.
Headquarters in Milan, Activities Worldwide
Group Highlights
- employees
> 4.5K
Total employees in 2024
- markets
~150
Total Markets in EMEA,
Americas and APAC Regions
- revenue 2024
2,341.6
Million Euros
- manufacturing
10
Production sites
- SUSTAINABLE GROWTH MODEL
We pursue a sustainable growth model by integrating social and environmental aspects into our corporate strategy.
- Patients
In 2024, over 100 million people benefitted from our Specialty & Primary Care portfolio.
And around 16.000 patients living with rare diseases received dedicated treatments from our Rare Diseases business.
Who We Are
Culture
Our Company Culture
The Recordati culture is built on the pillars of entrepreneurship, purpose and belonging. We strive to ensure everyone feels welcome, respected, and supported in their professional and intellectual growth, and appreciated for their uniqueness and diverse talent. To support our people, we continue to promote and build our culture, to allow people to be their full selves at work, feel safe to speak up and empowered to make decisions, experiment and innovate.
Our purpose:
Recordati. Unlocking the full potential of life.

Who We Are
People
“We know that everyone benefits if our people come to work every day feeling engaged. We all get more satisfaction from our work if we have opportunities to grow and develop, and generally feel included, safe and energised. And the company also benefits, as engaged employees contribute to stronger performance, helping us drive value and achieve our goals.”
Alessandra Abate
Group Chief People and Culture Officer
People Highlights
Our people are at the heart of our success. When employees feel engaged, supported, and empowered to grow, they bring greater energy, creativity, and commitment to their work. This not only enhances their own job satisfaction and well-being but also drives stronger performance across the company. That’s why we invest in opportunities for our people to develop, grow, and build fulfilling careers with us.
4.583
Total number of employees at end of FY2024 (3% growth vs. 2023)
50%
of all employees are women
52%
of total new hires in the year are women
95%
of employees hired on permanent contracts
146,000
Total training hours across the company
32
hours of training per person
12,650
hours of health and safety training
310
Top and Senior Managers
33.5%
of Top and Senior Management roles are held by women
42%
of women on the Board of Directors
People employed by region
63%
Europe
23%
Asia/Oceania
8%
Africa
6%
America
Career & Culture Initiatives
Employee Value Proposition
In 2024, we launched our employee value proposition (EVP); our promise to current and future employees of the unique value we offer in return for their skills, experience and commitment. The EVP slogan, which will be reflected in all touchpoints with current and future employees, is: Ready to Unlock Your Full Potential?
People Engagement
In 2024, we conducted a Culture Survey to understand how our company culture has evolved over the past few years. The results were very encouraging showing a continued positive trend in all dimensions year-on-year.
For more information about our People & Culture initiatives, visit our corporate website.
For more information, please refer to the Consolidated Sustainability Statement.
Who We Are
Business
Specialty & Primary Care
We have a strong and proven heritage of supporting people living with a wide range of common illnesses that affect large populations day-to-day. Across Specialty & Primary Care (SPC), we create value for patients, payers, and physicians with both prescription and self-medication treatments, with a focus on cardiovascular, urological, gastrointestinal and cough and cold therapy areas. We provide treatments globally, with direct presence in Europe and North Africa, and have proven experience in supporting access to medicines throughout their lifecycle.
Rare Diseases
Of the 7,000 known Rare Diseases, less than 10% have an available treatment option. Since 2007, through our dedicated business unit Recordati Rare Diseases, we’ve focused on helping the few who suffer from little known conditions.
Our mission is to reduce the impact of rare and devastating diseases by providing urgently needed medicines in key therapeutic areas including:
- Metabolic
- Endocrinology
- Hema-Oncology
Pharmaceutical Chemicals
We produce various active and intermediate ingredients that, in addition to meeting our own needs, we also sell to third-party pharmaceutical companies.
Geographical Presence
Present in around 150 countries with our SPC products and our RD treatments
- specialty and primary care
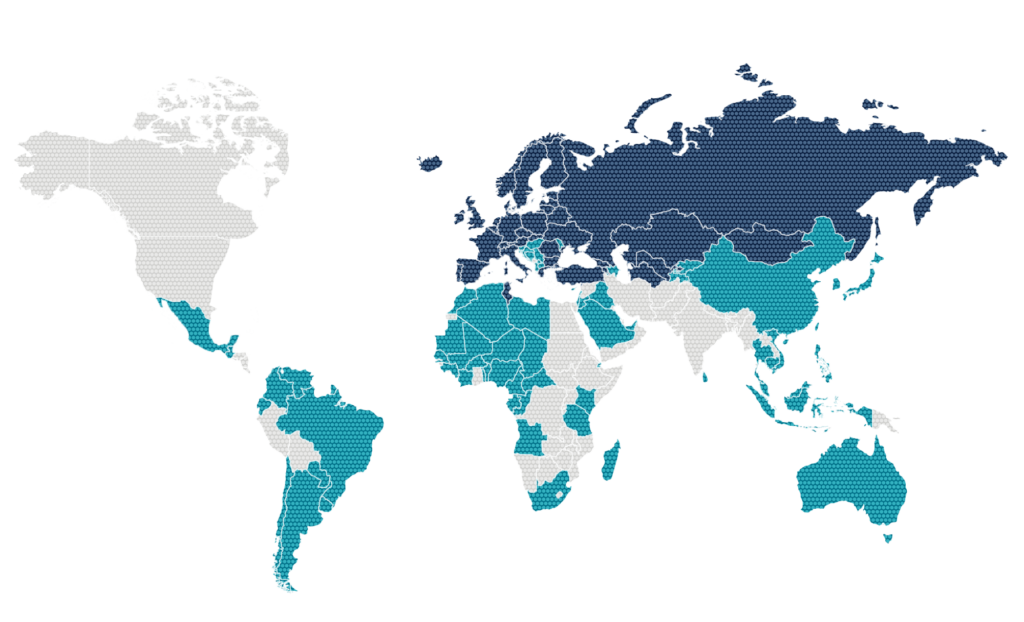
- Subsidiaries, branches, permanent establishment and direct promotion
- Licensees, distributors, commercial agreements
- rare diseases
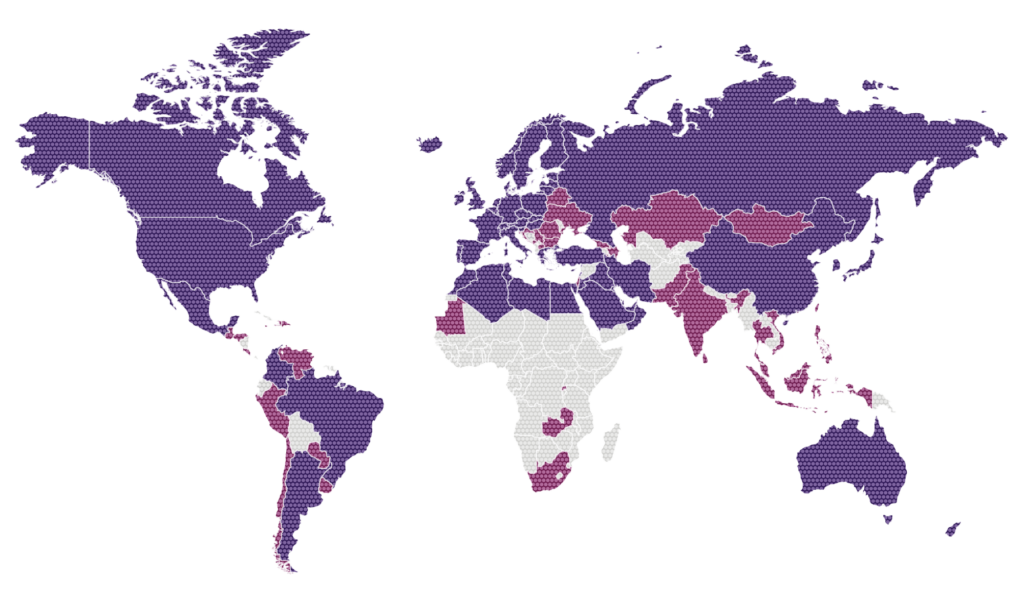
- Subsidiaries, branches, permanent establishment and direct promotion
- Licensees, distributors, commercial agreements
Who We Are
Industrial Operations
We operate several state-of-the-art production sites where the manufacturing of finished products and active ingredients has been closely linked with pharmaceutical activities since the beginning of the company’s history.
Production Sites
Our production sites are equipped with state-of-the-art installations and our research laboratories are fitted with the latest equipment.
All plants operate in full compliance with the environmental protection regulations and in compliance with the cGMP (current Good Manufacturing Practices).
- 7 Pharmaceutical Manufacturing plants
- 1 packaging and distribution centre
Dedicated to products for Rare Diseases
- 2 Pharmaceutical chemical plants
Who We Are
Research & Development
Product Pipeline & Future Developments
We are committed to continuing to bring innovation that benefits patients. We continuously develop new specialties originating either internally or acquired through development agreements with other pharmaceutical companies and research institutes. Commitment, scientific rigour, capability and highly-specialised personnel allow us to develop new treatments and build an innovative product pipeline.
- investment
286
(Million Euros) *
in Research & Development in 2024
- investment
+11.8%
in Research & Development versus FY 2023
For more information about our R&D projects, visit our corporate website.
*This amount includes amortisation relating to products acquired or for which the Group is a licensee
Portfolio
These are just a few of the important portfolio milestones achieved in 2024 that are enabling us to continue to support growing numbers of patients and their families.
Enjaymo®
Signifor®
Isturisa®
In China, our new drug application (NDA) for Isturisa® was approved by the country’s National Medical Products Administration (NMPA) in September. Isturisa® is used to treat adult patients living with Cushing’s Syndrome. In June 2024, in the U.S., the Isturisa® supplemental new drug application was submitted for Cushing’s Syndrome label extension and the FDA granted the expanded indication for Isturisa® in April 2025.